Oxford English Dictionary Error?
Joseph F. Alward
The OED says a siphon is a "...pipe....bent so that one leg is longer than the other, and used for drawing off liquids by means of atmospheric pressure, which forces the liquid up the shorter leg and over the bend in the pipe."
In an article published in "Physics Education," Dr. Stephen Hughes, a Queensland University of Technology Senior Lecturer in Physics, claims that the OED description of a siphon is incorrect. Gravity and the cohesive forces between water molecules are responsible for the operation of a siphon, not atmospheric pressure, he says.
A Wikipedia article is at odds with Dr. Hughes's explanation. In the Wikipedia article, the role of atmospheric pressure in the operation of a siphon is described, and the siphon's operation is shown not to rely on cohesive forces between water molecules. Click here to read the Wikipedia article.
Quoting from the Wikipedia article (parenthetical comments and underscore added):
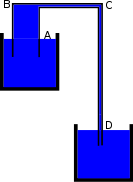
To demonstrate, the longer lower leg of a common siphon can be plugged at the bottom (see the figure at the right) and filled almost to the crest with liquid, leaving the top and the shorter upper leg containing only air at ambient pressure. When the plug is removed and the liquid in the longer lower leg is allowed to fall, it will cause a reduction of pressure at the top of the siphon, resulting in the liquid in the upper reservoir being pushed up into the reduced pressure area by atmospheric pressure acting on the upper reservoir. The liquid will then typically sweep the air bubble down and out of the tube and continue to operate as a normal siphon. As there is no contact between the liquid on either side of the siphon at the beginning of this experiment, there can be no cohesion between the liquid molecules to pull the liquid over the rise." |
 |
Demonstration
One can perform the demonstration described above with a flexible straw (see image below), a drinking glass, and a bowl. The glass should be taller than the bowl.

1. Fill a glass to the top with water, and place a shorter partially filled bowl, filled to within an inch of the top, next to it. The bowl should be wide enough to accommodate the four fingers of your hand.
2. Place your index finger under the bottom end of a flexible straw, place the top end of the straw in a narrow stream of water from a faucet, and fill it with water, being careful not to allow air bubbles to form.
3. With your finger blocking the bottom of the water-filled straw, squeeze out the water between the straw's flex and the top of the straw.
4. With your finger still sealing the bottom of the straw,
lower the bottom of the straw into the smaller bowl of water. Don't remove your finger, yet.
4. Bend the top of the straw (just the part containing air) into the water in the glass. Water will rise in the straw to the same level as the water in the glass, trapping an air bubble between it and the flex.
5. Now, without there being any initial contact between the water on either side of the bubble, i.e., without any cohesive force action, you're going to cause the water on the glass side of the straw to be pushed up the straw and over the flex, down into the bowl. Get ready.
6. Check to make sure that no part of the straw is pinched too tightly to allow water to flow past.
If it weren't for the force exerted by your index finger upward on the bottom of the column of water above the bowl, the water would be pulled downward by gravity, as well as pushed downward by the air pressure in the bubble above the column. If you were to remove your finger, the column of water would flow into the bowl, thereby increasing the volume of the bubble above it, reducing the air pressure in the bubble. We'll come back to that water in a moment.
Currently, the water in the part of the straw that's in the glass is in equilibrium. That means that the fluid pressure (atmosphere plus water) acting upward on the bottom of that column of water that's in the straw above the glass of water is balanced by the downward pull of the Earth (gravity) acting on that water and the downward push of the air pressure in the air bubble above that water. If the air pressure in the bubble is reduced, as it soon will be when you release your finger, the water in the straw above the glass will be pushed upward, over the bend, and into the bowl. Note that the force on the water in the straw is a push, not a pull. There is no pull by cohesive forces between water molecules because there is initially only air above the water, not water.
7. Release your finger from the end of the straw that's in the bowl and watch the water drain out of the glass and flow into the bowl. This happens without the need for any cohesive forces between water molecules.
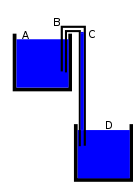
Hughes's Chain Link Model
The drawing in Figure A below, taken from the Wikipedia article, shows a functioning siphon consisting of a tube of uniform diameter (the diameter is the same on both sides). Figure B below illustrates the chain model that Dr. Hughes believes explains how the longer and heavier water column on the right side of a siphon pulls the shorter and lighter water column upward and over the hump. Thus, in Dr. Hughes's explanation of the siphon, it is gravity and chain-link forces (cohesive forces between water molecules), not atmospheric pressure, that is responsible for the operation of the siphon. I believe this explanation is incorrect, and I support my belief mainly by pointing to the arguments found in the Wikipedia article, partially reproduced below.
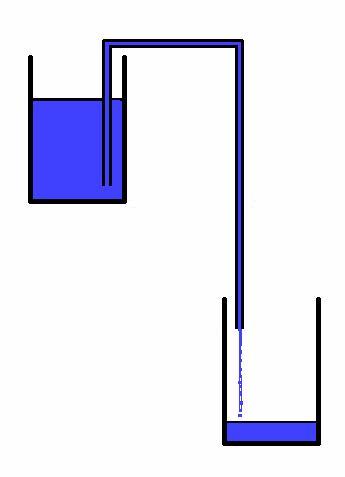 Figure A |
Figure B |
As the Wikipedia article points out, if the chain link model correctly described how the siphon works, then how could the siphon in Figure C below work?
Figure C |
We know that siphons such as the one on the left in Figure C work even if the diameter of the pipe on the left side is much greater than on the right side, because the greater area, multiplied by the combined fluid (air plus water) pressure, compensates for the greater water weight. Water is pushed upward and across the top and down to the right side. However, Dr. Hughes's chain-link gravity model would incorrectly predict that since the short but wider "chain" of water on the left side is heavier than the longer but narrower "chain" of water on the right side, the "falling" chain on the left would pull upward on the chain of water on the right side, lifting the water from the lower reservoir. That's not what happens. |
How Does the Siphon Work?
(The argument below is not from the Wikipedia article; it is the author's own. I would welcome any criticisms or comments.)
The drawing below describes how atmospheric pressure--not cohesive forces between water molecules--is responsible for pushing (not pulling!) the water in the shorter column up and over.
Figure D |
In Figure D on the left, as the column of water on the right side falls, the bonds between water molecules at B are continually breaking, which creates--for just an instant, a microscopic vacuum gap at B--a "cavitation"--in which the pressure is zero. In an instant after the cavitation is created at B, it is refilled with water, thus restoring the fluid pressure at that point.
This is how a siphon works, in my opinion. It works without the need for cohesive forces between water molecules.
|
Siphon Speed Does Not Depend on the Value of Atmospheric Pressure
Assuming that Dr. Hughes is wrong about cohesive forces being responsible for siphoning, and that Wikepedia and others, including myself, are not the ones instead who are mistaken, why might Dr. Hughes have reached the erroneous conclusion that siphoning does not depend on atmospheric pressure to work?
I think a clue to this misunderstanding might lie in the equation for the flow velocity. Applying Bernoulli's equation to the Figure E below, we find that the speed v of the fluid exiting the siphon is
v = (2gh)1/2
Equation 1
Figure E
where h is the distance between the top of the reservoir and the outlet of the siphon. This equation contains no reference to atmospheric presssure. The speed of the fluid does, indeed, not depend on the value of the atmospheric pressure. This is the equation Dr. Hughes refers to in his article, in which he states that the speed of the cannot depend on atmospheric pressure. But, as was made clear in the arguments above, without atmospheric pressure, the siphon does not work: cavitations formed at Point B in Figure D, for example, would shut down the flow. Once atmospheric pressure is restored, the cavitations disappear, siphoning resumes, and the speed of siphoning does not depend on the value of the atmospheric pressure. That's not the same as saying, of course, that siphoning of water doesn't depend on the existence of atmospheric pressure; without it, the siphon wouldn't work.
One has only to look again at the Wikipedia article to see that if atmospheric pressure was zero, the siphon would not work; the siphon speed would be zero. An equation derived in the Wikipedia article shows that the maximum siphon speed depends on the value of the atmospheric pressure.
v = [2(PATM /ρ-ghB)]1/2
Equation 2where PATM is the atmospheric pressure, hB (not shown) is the height above the surface of the upper reservoir of the high point on the siphon tube, and ρ is the density of water (1000 kg/m3) .
For example, if the siphon is at "sea level," where PATM = 101,000 Pa, and we assume hB is ignorably small, the maximum exit speed in a siphon is about 14 m/s. Under these conditions, a siphon could have an exit speed between zero and 14 m/s, depending on the value of h. The maximum speed would be obtained if h were about 10 meters.
If the atmospheric pressure were one-fourth of this, the maximum exit speed would be about 3.5 m/s. If the atmospheric pressure were zero (assuming hB= 0), the maximum speed of the siphon would be zero.
Clearly, the operation of the siphon depends very much on the atmospheric pressure, insofar as its value determines the maximum siphon speed.
Address comments and questions to jfalward@aol.com.